Memorizing the Periodic Table
I think the only reason I do it, is to see if I still can.
Tom Lehrer on singing the Elements Song.
Updates | Online Recitation Tester
Introduction
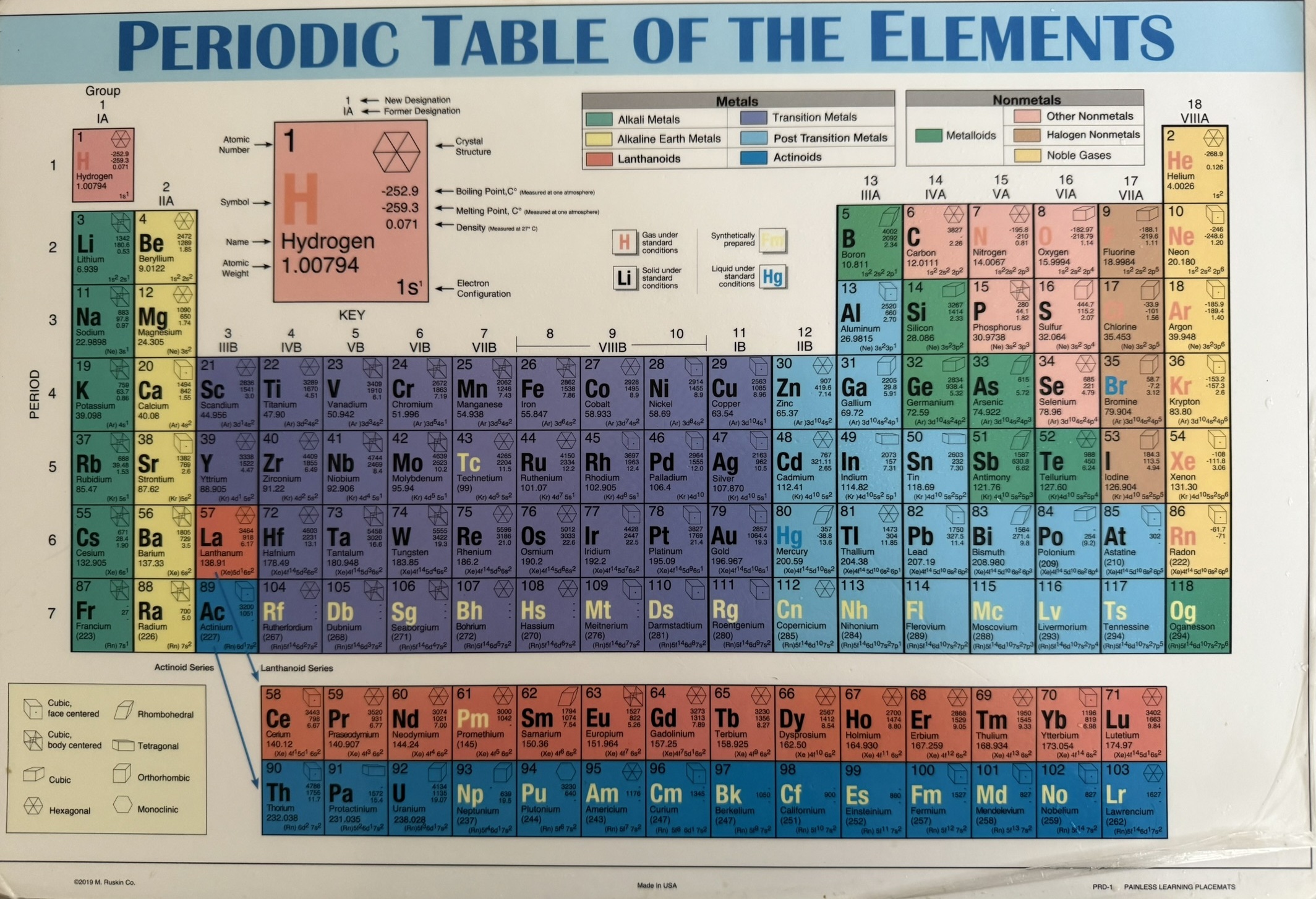
I’ve eaten all my meals at home the last twenty years off a placemat printed with the periodic table, Figure 1. It has all the usual details—atomic weights, numbers, electron configurations, densities, melting and boiling points, metals vs nonmetals, and period and group labels. I’ve looked at it on and off countless times, picking up fragments of understanding. But beyond hydrogen, helium, lithium, and beryllium, I struggle. I know other elements, and can place them roughly, but I have no real sense of how electron configurations map to the table, or what that parade of letters and numbers—1s² 2s² 2p⁶, and so on to d and f—really mean.
In July 2025, Tom Lehrer died. Famous for many things, but for me most as the singer of the Periodic Table Song. His death prompted me to listen again, and to wonder if I could memorize the elements? Since high school I’ve struggled with rote learning, especially my nemesis, French vocab. But what if I tried to memorize the periodic table? Lehrer doesn’t sing the elements in a recognizable order. That makes it harder: the table’s structure gives clues about where things belong, unlike a list of 103 names. And the table’s framework is easy to figure out knowing just a few tricks—the 1-3-5-7 pattern, spins up and down. That realization set me off on my current project: to memorize the periodic table up to element 103, Lawrencium. Lr was the end of the line when I was a kid; it’s all made up nonsense from thereon 🙂, but see Section 2.
This post is a collection of tips, tricks, and facts that have helped in my figuring and memorizing journey. Here is my progress to date—my last self-test.
Updates
Update 2025-09-05: Current pace > 5 minutes. Creating chunks and quatrains to build fluency.
Update 2025-09-04: Practicing recitations, focusing on the “carriage returns” today, Figure 2.
Suffering as playing anomia?!
- anomia (noun)
- Difficulty in naming objects or persons, as a form or symptom of aphasia;
Update 2025-09-03: Wrote an online elements recitation tester to test and time myself reciting the elements in order. Benchmark time: Tom Lehrer sings all the elements in under 60 seconds! My goal: under 2 minutes.
Update 2025-08-25: Decided to go full monty and number 118, Organesson. See Section 9 for details of the later synthetic elements.
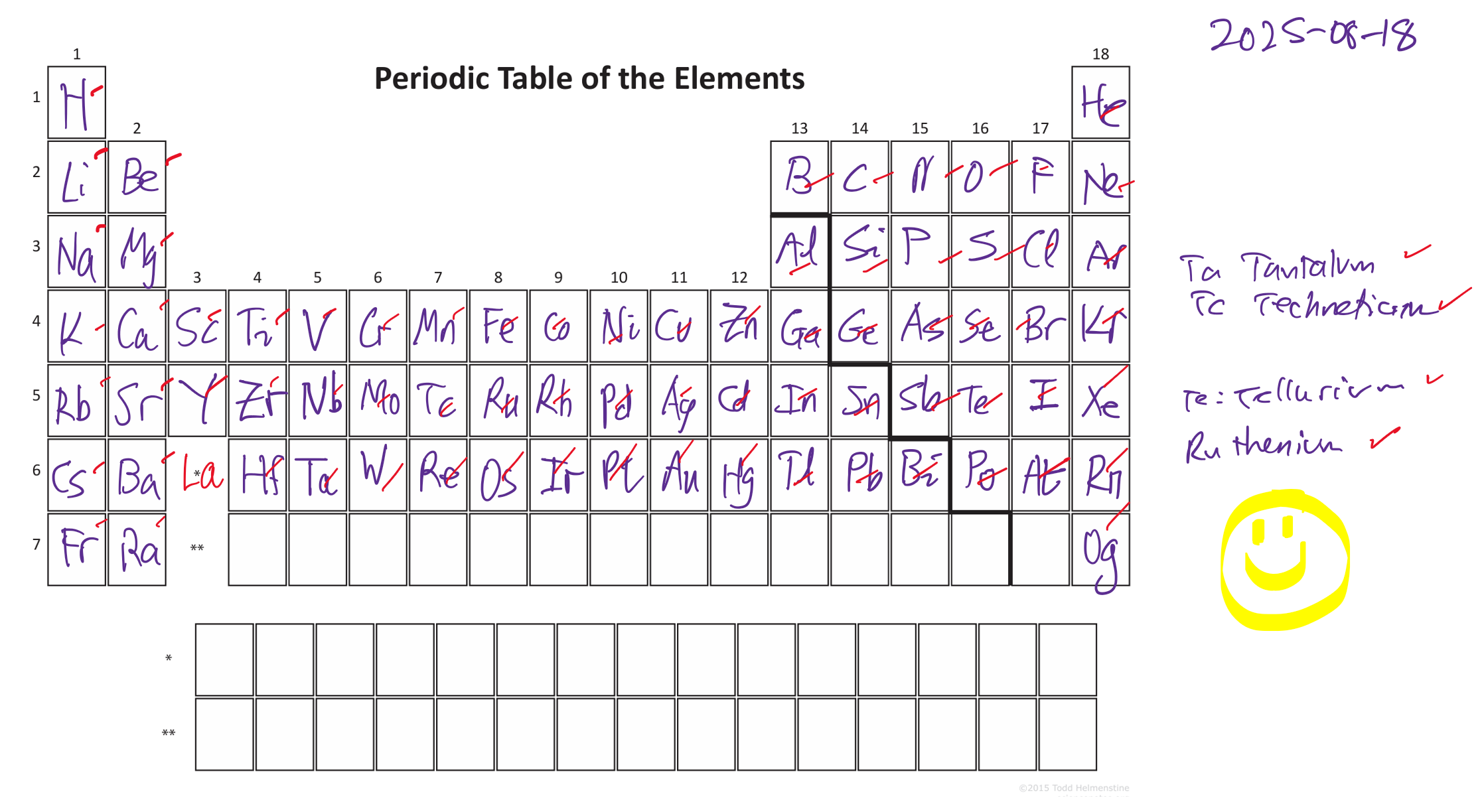
Update 2025-08-21: wrote out the table from a blank sheet of paper, Figure 4! Observations on my memorization process. First, knowing something about the names is a huge help, holmium from Stockholm, lutetium from Latin Paris, Samarium from Russian offician Samarsky, Ruthenium from Latin Russia. Abstract words (like names, no story) I find really hard, the extra context helps. Second, the symbols are a harder than the names when they don’t follow the standard pattern. Third, my brain seems to refuse to remember things it can work out, like the atomic numbers—draw the boxes and count! Fourth, order matters. I can go down groups 1 and 2 and the noble gasses. Do the p block top row then by column. The d block is very relational and I need to draw it out to get hints vertically and horizontally at the moment. The Lanthaides and Actinides work best in groups of seven. Next step: recite like a poem in atomic number order with no hesitations. Overall, easier than I expected but then I’m not remembering them for a (French vocab) test tomorrow.
Update 2025-08-18: Good progress.
General Rules
Two-letter symbols usually take the first one or two letters of the English name (He, Li, Ne, Na, Al, Si). When the first two letters are already taken by another element, the symbol may use another distinctive pair from the name, such as Rn for radon given Ra radium. Using As for arsenic was set before (and allows) Ar for argon. Sometimes its just disambiguation or historical precedent: Mg for magnesium instead of the expected Ma (not used); Mn for manganese; Mo for molybdenum. It is helpful to have some idea of the order of element’s discovery.
Elements discovered in antiquity often have Latin or Greek names.
- Na = natrium (sodium)
- K = kalium (potassium)
- Fe = ferrum (iron)
- Cu = cuprum (copper → Cyprus a major source)
- Ag = argentum (silver → Argentina)
- Sn = stannum (tin)
- Sb = stibium (antimony, from stibnite Sb₂S₃ ore; possibly “against solitude,” from Greek anti+monos (alone))
- Au = aurum (gold)
- Hg = hydrargyrum (mercury)
- Pb = plumbum (lead)
- W = wolfram (tungsten)
If two names would clash, the less common one gets a less intuitive symbol. Example: carbon = C, so cobalt = Co (not C). New synthetic elements generally follow the pattern of taking the first letters of their names (e.g., Rf = rutherfordium, Ds = darmstadtium).
There are other geographic associations (country → name): Germany (Germania) gave its name to germanium, Poland to polonium, France to francium, Scandinavia to scandium, more broadly Europe to europium, and more locally, Ytterby, Sweden to Y, Yb, Er, Tb. Finally, America to americium.
Fourteen Single-letter Elements
H B C N O F P S K V Y I W U
Three d Orbital R elements
- Re = Rhenium
- Group 7, period 6, below Mn, Tc.
- Discovered 1925 in Germany, named after the Rhine River. The last stable element discovered in nature.
- Heavy, rare metal, used in superalloys (jet engines, turbines).
- Think: Re = Rhine = Rhenium.
- Ru = Ruthenium
- Group 8, period 5, between Fe and Os.
- Discovered 1844 in Russia, named after Ruthenia (old Latin name for Russia/Ukraine).
- A platinum-group metal, used in electronics and catalysts.
- Think: Ru = Ruthenia (Russia) = Ruthenium.
- Adds ut into Rhenium
- Rh = Rhodium
- Group 9, period 5, between Co and Ir.
- Discovered 1803 in England, name from Greek rhodon (“rose”), because of red salts.
- Very shiny, silvery, used in catalytic converters.
- Expensive and hard.
- Think: Rh = Rose = Rhodium.
Four p and d Orbital T Elements
- Ta = Tantalum
- Group 5, period 6, below V and Nb.
- Name: from King Tantalus in Greek myth—the element “refused to drink” acid, just as Tantalus was tormented by water he couldn’t drink.
- Uses: corrosion-resistant alloys, capacitors.
- Think: Ta = Tantalus (thirsty but can’t drink).
- Tc = Technetium
- Group 7, period 5, between Mn and Re.
- Name: from Greek technetos (“artificial”), because it was the first man-made element (1937). Traces occur in U ores.
- No stable isotopes; used in medical imaging.
- Think: Tc = Technetium = Tech-made.
- Tl = Thallium
- Group 13, period 6 between Hg and Pb, below Ga and In.
- It has two valence states (I and III), which is why it shows up in poison/biological confusion, see Section 16.
- Name: from Greek thallos (“green shoot”), due to green spectral line.
- Toxic, once used in rat poison.
- Think: Tl = Thallium = Thallus = Green shoot.
- Te = Tellurium
- Group 16, period 5, chalcogen family, below S and Se.
- Name: from Latin tellus (“earth”).
- Metalloid, used in semiconductors and solar panels.
- Think: Te = Tellurium = Tellus = Earth.
The Lanthanides
The Lanthanide Series (57–71, La → Lu) are the rare earths, the 15 elements that fill the 4f orbitals. They sit in period 6, between Ba and Hf. They are chemically very similar (mostly trivalent metals, silvery/soft, reactive in air), which makes them hard to separate—and hard to memorize.
- La = Lanthanum
- Name: Greek lanthanein (“to lie hidden”). First of the series.
- Soft, silvery, used in camera lenses (high refractive glass), catalysts in petroleum refining.
- Think: La = Lanthanum = Lurking/Hidden.
- Ce = Cerium SEER-ee-uhm
- Named after asteroid Ceres (discovered 1801, element in 1803).
- Commonest lanthanide, used in lighter flints, glass polishing, catalytic converters.
- Think: Ce = Ceres = Cerium.
- Pr = Praseodymium Pra/se/o/dym/i/um pray-zee-oh-DIM-ee-uhm
- Greek prasios didymos = “green twin.”
- Used in green glass and alloys, e.g., in aircraft engines (Pr–Nd alloys).
- Think: Pr = Prase = green twin.
- Nd = Neodymium Ne/o/dym/i/um nee-oh-DIM-ee-uhm
- Greek neos didymos = “new twin.”
- Famous for super-strong Nd–Fe–B magnets, used in lasers, and glass tints (purple).
- Think: Nd = Neo twin = magnets.
- Pm = Promethium pruh-MEE-thee-uhm
- Named after Prometheus (stole fire).
- Radioactive, no stable isotopes. Used in luminous paint and nuclear batteries.
- Think: Pm = Prometheus = stolen fire.
- Sm = Samarium suh-MAIR-ee-uhm
- Named after mineral samarskite, itself from Russian official Samarsky.
- Used in Sm–Co magnets, and control rods in nuclear reactors.
- Think: Sm = Samarium = Samarsky.
- Eu = Europium yuh-ROH-pee-uhm
- Named for Europe.
- Used in red phosphors (TVs, LEDs), anti-counterfeiting in euro notes.
- Think: Eu = Europe = red glow.
- Gd = Gadolinium
- Named for Finnish chemist Gadolin.
- Used in MRI contrast agents, nuclear reactor neutron absorber.
- Think: Gd = Gadolin = medical scans.
- Tb = Terbium
- Named after Ytterby, Sweden (like Y, Er, Yb).
- Green phosphors in lighting, magneto-optic devices, added to magnets.
- Think: Tb = Terbium = Ytterby green.
- Dy = Dysprosium
- Greek dysprositos = “hard to get.”
- Added to Nd magnets for high-temperature strength, neutron absorber.
- Think: Dy = Difficult = Dysprosium.
- Ho = Holmium
- Named after Holmia, Latin for Stockholm.
- Strongest magnetic moment of any element, used in lasers, nuclear control rods.
- Think: Ho = Holmia/Stockholm = magnets.
- Er = Erbium
- Also from Ytterby, Sweden.
- Used in fiber-optic amplifiers, pink glass, lasers.
- Think: Er = Erbium = pink glass, lasers.
- Tm = Thulium
- Named for Thule (mythic far north).
- Rarest stable lanthanide, used as a portable X-ray source and in lasers.
- Think: Tm = Thule = rare north.
- Yb = Ytterbium
- From Ytterby, Sweden.
- Used in lasers, stress gauges, some electronics.
- Think: Yb = Ytterby = lasers.
- Lu = Lutetium loo-TEE-shee-uhm
- Named after Lutetia (old name for Paris).
- Hardest, densest lanthanide; used in PET scan detectors (Lu₂SiO₅ scintillators) and catalysts.
- Think: Lu = Lutetia/Paris = heavy end.
Sc and Y are often also called rare earths because they occur in the same ores and behave similarly.
The magnetic quartet consists of Nd = Neodymium, the asis of Nd₂Fe₁₄B magnets (neodymium-iron-boron) and the strongest permanent magnets known. Sm = Samarium, the basis of SmCo magnets (samarium-cobalt), which are very strong and more temperature-stable than Nd magnets. Dy = Dysprosium, added to Nd magnets to improve high-temperature performance. Tb = Terbium is a crucial component in high-performance magnets used in various military applications, particularly in defense systems. These magnets, often neodymium-iron-boron (NdFeB) based, are vital for aircraft, submarines, and missiles. The element enhances the magnets’ thermal stability but is very expensive.
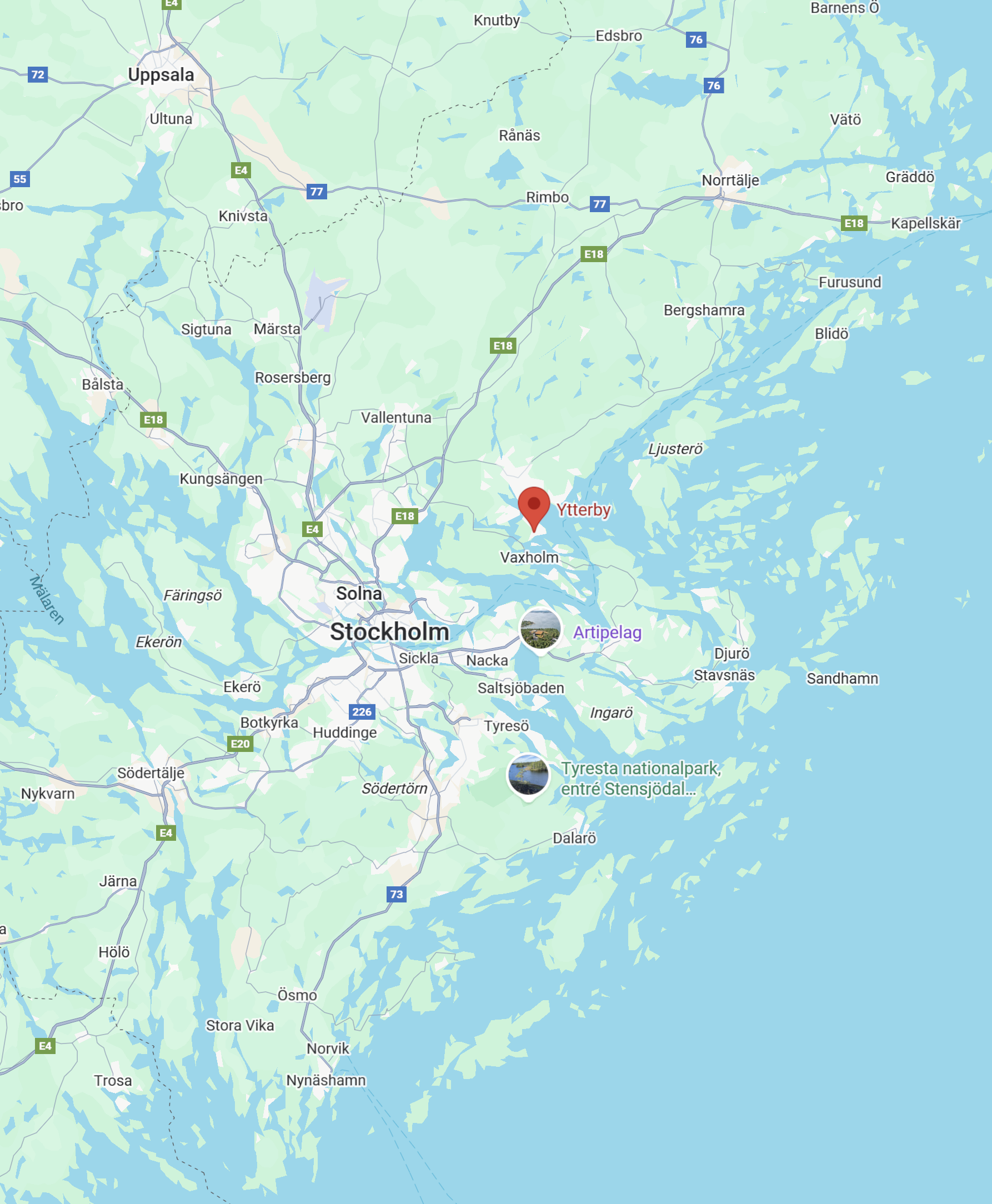
Ytterby Sweden
Ytterby is a small village on the island of Resarö in the Stockholm archipelago, Sweden, with an outsized role in the history of chemistry.
The village is famous for the Ytterby mine, a quartz and feldspar mine that turned out to be one of the richest sources of rare earth elements ever found. In the late 18th and 19th centuries, chemists analyzing its ores discovered a remarkable series of new elements. In fact, more chemical elements were first identified from Ytterby than from any other single location in the world. Notably:
- Yttrium (Y) – named directly after Ytterby (discovered 1794).
- Terbium (Tb) – also from “Ytterby”.
- Erbium (Er) – likewise.
- Ytterbium (Yb) – also named after the mine.
In addition, the mine’s minerals also yielded:
- Scandium (Sc)
- Holmium (Ho) (named after Stockholm)
- Thulium (Tm) (named after the legendary Thule, i.e., Scandinavia)
- Gadolinium (Gd)
The mine is in a granitic pegmatite dike intruding into older rocks. Pegmatites are very coarse-grained igneous (crystallized from molten rock) rocks formed late in the cooling of a magma body. Because they crystallize from the last, water- and element-rich fraction of the melt, they tend to concentrate rare and unusual elements that don’t fit well into the main rock-forming minerals.
At Ytterby, this pegmatite carried feldspar, quartz, mica, and importantly, complex oxides and silicates of rare earth elements. Minerals like gadolinite, euxenite, and fergusonite turned up there. These contained yttrium and the other “rare earths” (lanthanides plus Sc, Y).
The lanthanides are chemically very similar to each other, which is why they occur together in the same minerals and were so hard to separate. The Ytterby pegmatite was unusual because it was both rich enough and accessible enough that mineral collectors brought samples to chemists in Uppsala and elsewhere, who painstakingly teased apart the new elements over the 19th century.
So it wasn’t that Ytterby had a unique element never seen elsewhere; it was that the pegmatite concentrated the rare earths to a degree and purity that made systematic discovery possible at that time.
| Mineral (from Ytterby pegmatite) | Chemist (date) | Element(s) isolated/named |
|---|---|---|
| Gadolinite (“ytterbite”) | Johan Gadolin (1794, Åbo/Turku, Finland) | Yttrium (Y) – named for Ytterby |
| Later also source of Erbium (Er) and Terbium (Tb) via Carl Gustaf Mosander (1843, Stockholm) | ||
| Ytterite / xenotime (yttrium phosphate) | Carl Gustaf Mosander (1843) | Terbium (Tb) and Erbium (Er) separated from yttrium |
| Ytterbite / gadolinite residues | Jean Charles Galissard de Marignac (1878, Geneva) | Ytterbium (Yb) |
| Euxenite, fergusonite | Lars Fredrik Nilson (1879, Uppsala) | Scandium (Sc) (named for Scandinavia) |
| Per Teodor Cleve (1879, Uppsala) | Holmium (Ho) (“Holmia,” Latin for Stockholm) and Thulium (Tm) (from Thule, a poetic name for Scandinavia) | |
| Jean Charles Galissard de Marignac (1880) | Gadolinium (Gd) (honoring Gadolin) |
The Actinides
The actinides (89–103, Ac → Lr) are the 5f block elements. They are highly radioactive and most synthetic beyond uranium.
- 89 Ac = Actinium
- Name: from Greek aktinos (“ray”), because it glows strongly.
- First non-primordial actinide discovered (1899).
- Uses: glowing salts (old radium substitutes), cancer therapy research.
- Think: Ac = Actinium = rays.
- 90 Th = Thorium
- Named for Norse god Thor.
- Discovered 1828; relatively abundant.
- Used in gas mantles (historic) and as a potential thorium reactor fuel.
- Think: Th = Thorium = Thor’s fuel.
- 91 Pa = Protactinium
- Name: from Greek protos (“before”), because it comes before actinium in decay chains.
- Extremely rare, discovered 1913.
- Studied for nuclear science only; very radioactive.
- Think: Pa = Protactinium = proto-actinium.
- 92 U = Uranium
- Named after planet Uranus (discovered just before the element, 1781/1789).
- Famous as nuclear fuel and for weapons; also used in dating rocks (U–Pb).
- Think: U = Uranium = Uranus.
- 93 Np = Neptunium
- Named after planet Neptune (next after Uranus).
- First transuranic element (discovered 1940).
- Reactor by-product; precursor to Pu-238.
- Think: Np = Neptunium = after Uranium, like Neptune after Uranus.
- 94 Pu = Plutonium
- Named after Pluto (then a planet, following the sequence Uranus–Neptune–Pluto).
- Discovered 1940; key to nuclear weapons and space RTGs (radioisotope thermoelectric generators).
- Think: Pu = Plutonium = Pluto’s deadly power.
- 95 Am = Americium
- Named for the Americas, by analogy to europium (Eu 63 above it).
- Discovered 1944.
- Used in smoke detectors (Am-241), neutron sources.
- Think: Am = Americium = American smoke alarms.
- 96 Cm = Curium
- Named after Marie and Pierre Curie.
- Discovered 1944.
- Used in RTGs for spacecraft.
- Think: Cm = Curium = Curie power.
- 97 Bk = Berkelium
- Named after Berkeley, California (discovered there in 1949).
- Purely a lab element.
- Think: Bk = Berkelium = Berkeley lab.
- 98 Cf = Californium
- Named after California (1950).
- Strong neutron emitter, used in oil exploration, reactor startup, cancer therapy.
- Think: Cf = Californium = California neutron source.
- 99 Es = Einsteinium
- Named for Einstein.
- Discovered in debris from the first hydrogen bomb (1952).
- Purely research, helped discover Md.
- Think: Es = Einsteinium = H-bomb Einstein.
- 100 Fm = Fermium
- Named for Enrico Fermi, also from H-bomb debris (1952).
- Research only.
- Think: Fm = Fermium = Fermi’s H-bomb child.
- 101 Md = Mendelevium
- Named for Mendeleev, discovered 1955.
- First element synthesized atom-by-atom.
- Research only.
- Think: Md = Mendelevium = Mendeleev honored.
- 102 No = Nobelium
- Named for Alfred Nobel, discovered 1958–1966 (disputed, but settled).
- Research only, short half-lives.
- Think: No = Nobelium = Nobel prize element.
- 103 Lr = Lawrencium
- Named for Ernest Lawrence (inventor of the cyclotron).
- Discovered 1961.
- Reseach, end of the actinide series.
- Think: Lr = Lawrencium = Lawrence’s cyclotron.
Remember the groupings: the Planet Trio: Uranium (U), Neptunium (Np), Plutonium (Pu); the American Run: Americium (Am), Curium (Cm), Berkelium (Bk), Californium (Cf); and the Science heroes: Einsteinium, Fermium, Mendelevium, Nobelium, Lawrencium.
The Transactinites
Having now memorized the elements up to lawrencium (103), I decided to push all the way to 118 and remember the transactinides (104-118, Rf → Og). These last fifteen elements are all are synthetic, made in labs, and exist only for fleeting moments.
- Actinium (89, Ac) or Lawrencium (103, Lr) for context and numbering.
Rutherfordium (104, Rf) – First of the transactinides, claimed by both Dubna (1964) and Berkeley (1969); name proposed by Berkeley. Named after Ernest Rutherford, the New Zealand–born physicist who explained nuclear structure. Unusual in that an element is named after someone from outside the “big labs” (Rutherford died in 1937). Behaves like hafnium.
Dubnium (105, Db) – 1968–70, again disputed between Dubna and Berkeley. Eventually named after Dubna, the Russian town that hosts the Joint Institute for Nuclear Research. “Dubnium” was a compromise: the Berkeley team had suggested “hahnium” after Otto Hahn, but the international committees decided to recognize Dubna’s contribution. Falls below tantalum.
Seaborgium (106, Sg) – 1974, Berkeley clear discoverer. Named for Glenn T. Seaborg, co-discoverer of plutonium and many other actinides, Nobel laureate, and long-time leader of U.S. nuclear chemistry. Controversial because Seaborg was still alive at the time, but it set the precedent later followed with oganesson. Chemistry matches tungsten.
Bohrium (107, Bh) – 1981, Darmstadt. Named for Niels Bohr, the Danish physicist whose model of the atom remains iconic. “Bohrium” rather than “nielsbohrium” was chosen to keep the name concise. Very short-lived; behaves like rhenium.
Hassium (108, Hs) – 1984, Darmstadt. Named for Hesse, the German state where Darmstadt is located. The discoverers used the Latin name Hassia as the stem, hence hassium (Hs) rather than “hessium.” This mirrors other Latinized place-names (e.g., lutetium from Lutetia for Paris). Forms a volatile tetroxide like osmium.
Meitnerium (109, Mt) – 1982, Darmstadt. Named for Lise Meitner, Austrian-born Jewish physicist who co-discovered nuclear fission with Otto Hahn but was denied the Nobel. She worked much of her career in Berlin before fleeing Nazi Germany. This was the first element named after a woman scientist. Predicted to behave like iridium.
Darmstadtium (110, Ds) – 1994, Darmstadt. Named after the host city itself, continuing the tradition of tying discoveries to place as well as people. The Latin-style suffix “-ium” is used even though the name comes from a modern city. A platinum analogue.
Roentgenium (111, Rg) – 1994, Darmstadt. Named for Wilhelm Röntgen, the German physicist who discovered X-rays in 1895. The “oe” spelling reflects his actual name; the element name was standardized as “roentgenium” in English. Gold-like chemistry expected. Last of transition metals.
Copernicium (112, Cn) – 1996, Darmstadt. Named for Nicolaus Copernicus, the Polish astronomer who proposed the heliocentric model. The discoverers originally suggested “copernicum,” but the International Union of Pure and Applied Chemistry (IUPAC) insisted on “copernicium” for consistency. A volatile metal, perhaps liquid like mercury. First post-transition metal. End of d block.
- Nihonium (113, Nh) – 2003, RIKEN (Japan). First element discovered in Asia. “Nihon” is one of the Japanese words for Japan (“the land of the rising sun”). The name reflects national pride; the alternative “japonium” had once been proposed historically for element 43. In the thallium group. In p block.
Flerovium (114, Fl) – 1998–99, Dubna with Livermore. Named for Georgy Flerov, the Russian physicist who discovered spontaneous fission and pushed the Soviet heavy-element program. The name also honors the Flerov Laboratory of Nuclear Reactions at Dubna. Longer-lived isotopes hint at the theorized “island of stability.”
Moscovium (115, Mc) – 2003, Dubna–Livermore collaboration. Named for Moscow region, recognizing the local support for Dubna’s research infrastructure. Like dubnium, this is one of several elements named after Russian places. Like bismuth, but short-lived.
Livermorium (116, Lv) – 2000, Dubna–Livermore. Named after Lawrence Livermore National Laboratory in California, recognizing the U.S. partners in the discovery. One of the rare cases of a lab, rather than a city or scientist, lending its name. In the polonium group.
Tennessine (117, Ts) – 2010, Dubna with Oak Ridge and Livermore. Named after the U.S. state of Tennessee, home to Oak Ridge National Laboratory, which produced the crucial berkelium targets. The “-ine” ending follows the halogen group (fluorine, chlorine, etc.).
- Oganesson (118, Og) – 2002–2006, Dubna–Livermore. Named for Yuri Oganessian, Russian physicist who led superheavy element research for decades. Only the second time an element was named after a living scientist (after seaborgium). The “-on” ending matches the noble gases, though oganesson is expected to be a reactive solid rather than an inert gas.
Note grouping by discovers:
Di Di US D D D D D D J Jt Jt Jt Jt Ru
- Rf 104 to Sg 106:
- US proposed rutherfordium
- Russia proposed dubnium
- Seaborgium US clear priority
- Bh 107 to Cn 112: Germany, GSI Darmstadt, recognized discoverer
- Nh 113 Japan, RIKEN
- 114-117 true collaboration: Dubna’s accelerators and Livermore/Oak Ridge’s targets and analysis; names split 2+2:
- Fl 114 to Mc 115: Russia
- Lv 116 to Ts 117: US
- Og 118: Russia
The stories of these discoveries are as much about the laboratories as the elements. The Joint Institute for Nuclear Research (JINR) in Dubna, Russia, with its Flerov Laboratory of Nuclear Reactions led by Georgy Flerov and later Yuri Oganessian, was a powerhouse, especially in collaboration with the United States. Lawrence Berkeley National Laboratory (LBNL) in California, founded by Ernest Lawrence, had earlier produced many transuranics under Glenn Seaborg and Albert Ghiorso. Its sister, Lawrence Livermore National Laboratory (LLNL) east of San Francisco, partnered with Dubna on elements 114–118. In Germany, the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt (the original Gesellschaft für Schwerionenforschung) used its UNILAC (Universal Linear Accelerator) and SHIP (Separator for Heavy Ion reaction Products) to claim 107–112 under leaders such as Peter Armbruster and Sigurd Hofmann. Japan entered the race when RIKEN (Rikagaku Kenkyūjo, Institute of Physical and Chemical Research) in Wako, at its Nishina Center, produced Nihonium under Kosuke Morita. And Oak Ridge National Laboratory (ORNL) in Tennessee supplied the rare berkelium targets needed for Tennessine.
The motivation has always been partly fundamental—can we make the heaviest possible nucleus, and how do the rules of nuclear structure and relativistic chemistry hold up?—and partly practical, in the form of new accelerators, detectors, and methods for handling exotic isotopes. The elements themselves are too short-lived to be useful, but the science behind them has strengthened nuclear medicine, isotope production, and our understanding of the limits of matter.
Nobel recognition has been rare. Glenn Seaborg shared the 1951 Chemistry prize for his earlier transuranium work, and many others received national honors, but the later generations of element discoverers—whether in Dubna, Darmstadt, or Japan—have so far not been recognized with Nobels. The names themselves, however, stand as monuments: Rutherford, Seaborg, Meitner, Oganessian, and the places that produced them.
Fabrication
Making superheavy elements is about fusing two nuclei together, which is a balancing act between bringing them close enough to fuse and not blowing them apart instantly. The basic method is called heavy-ion fusion. An accelerator beam of relatively light, neutron-rich ions (like calcium-48) is accelerated to very high speeds. The target is a thin film of a heavier actinide (curium, berkelium, californium, plutonium, etc.), prepared on a backing foil. In a fusion attempt the beam nuclei smash into the target nuclei; most scatter or bounce, but very rarely they “stick” together and form a compound nucleus. The evaporation residue—the fused nucleus—is extremely excited, and it usually cools by “evaporating” 2–4 neutrons. The surviving atom is the new element. Separation and detection involves specialized separators (like GSI’s SHIP or Dubna’s gas-filled recoil separator) to filter out the fused nuclei from the overwhelming background. Detectors then watch for characteristic alpha decay chains (sequential emission of α particles with known energies).
There are two fusion approaches. Cold fusion (GSI Darmstadt, 1980s–1990s) uses lead-208 or bismuth-209 targets with medium projectiles (chromium, zinc, nickel) and produced elements 107–112. It is called “cold” because the compound nucleus carried less excitation energy, leading to evaporation of only 1–2 neutrons and slightly longer half-lives. Hot fusion (Dubna, 1990s onward) uses actinide targets (curium, californium, berkelium) with calcium-48 beams. Calcium-48 is doubly magic (20 protons, 28 neutrons), unusually stable for such a neutron-rich nucleus, and “sticky” in fusion. Hot fusion produced elements 113–118. Their half-lives are short, but this method gave higher production cross-sections.
Fabrication is hard. The fusion probability (cross-section) is minuscule, sometimes only one atom per week or month and competing processes such as fission and scattering destroy almost every attempt. The targets must be isotopically pure and can weigh just nanograms to milligrams. Producing enough berkelium, for example, required a year’s irradiation at Oak Ridge. Detection relies on correlated decay chains: you never “see” the atom itself, only its series of α decays ending in known isotopes.
Benefits of this work include developing detectors that can see a single atom. It has pushed isotope production and target technology to extremes. The “calcium-48 trick” was a breakthrough that tipped the balance toward Dubna in the late 1990s and 2000s.
Flerovium (114)
Some isotopes of flerovium, especially flerovium-289 and flerovium-290, live for a few seconds—surprisingly long compared to neighboring superheavies. This unusual stability was taken as tantalizing evidence for the predicted “island of stability,” a region around proton numbers 114–126 and neutron number about 184 where nuclear shell effects might give half-lives of minutes or longer. Although flerovium did not quite reach that promise, its behavior confirmed that shell closures at high Z really do make a difference.
Oganesson (118)
Oganesson, element 118, was first synthesized between 2002 and 2006 in experiments at Dubna in Russia, working in collaboration with Lawrence Livermore in the United States. The target was californium-249, itself a man-made actinide prepared in milligram quantities at Oak Ridge, deposited as a thin layer on a titanium backing. The beam was calcium-48, a “doubly magic” nucleus with 20 protons and 28 neutrons, unusually stable for such a heavy isotope. When a calcium nucleus fused with a californium nucleus, the result was a highly excited compound nucleus that quickly cooled by evaporating three neutrons to give oganesson-294. The production rate was vanishingly small, perhaps one atom per month of beam time, and each atom survived less than a millisecond before alpha decaying. The atoms recoiled from the target and were separated from the background by a gas-filled recoil separator, then implanted into silicon detectors that recorded their alpha decay chains. These chains led step by step down to known isotopes, providing a fingerprint that the parent had indeed been oganesson. After further confirmations in 2005–06, the discovery was accepted, and in 2016 the International Union of Pure and Applied Chemistry approved the name oganesson in honor of Yuri Oganessian, the Russian physicist who had long championed and led this line of research.
THE ELEMENTS by Tom Lehrer
words: Tom Lehrer
music: “The Major General’s Song” from The Pirates of Penzance, by Arthur Sullivan (public domain)
There’s antimony, arsenic, aluminum, selenium,
And hydrogen and oxygen and nitrogen and rhenium,
And nickel, neodymium, neptunium, germanium,
And iron, americium, ruthenium, uranium,
Europium, zirconium, lutetium, vanadium,
And lanthanum and osmium and astatine and radium,
And gold and protactinium and indium and gallium,
And iodine and thorium and thulium and thallium.
There’s yttrium, ytterbium, actinium, rubidium,
And boron, gadolinium, niobium, iridium,
And strontium and silicon and silver and samarium,
And bismuth, bromine, lithium, beryllium, and barium.
There’s holmium and helium and hafnium and erbium,
And phosphorus and francium and fluorine and terbium,
And manganese and mercury, molybdenum, magnesium,
Dysprosium and scandium and cerium and cesium.
And lead, praseodymium and platinum, plutonium,
Palladium, promethium, potassium, polonium,
And tantalum, technetium, titanium, tellurium,
And cadmium and calcium and chromium and curium.
There’s sulfur, californium and fermium, berkelium,
And also mendelevium, einsteinium, nobelium,
And argon, krypton, neon, radon, xenon, zinc and rhodium,
And chlorine, carbon, cobalt, copper, tungsten, tin and sodium.
These are the only ones of which the news has come to Ha’vard,
And there may be many others but they haven’t been discavard.
Note:
There exists a much earlier version of this song.
The complete lyrics, ’Which are by Aristotle, are:
There’s earth and air and fire and water.
The Elements Alphabetically
- Actinium, Aluminum, Americium, Antimony, Argon, Arsenic, Astatine
- Barium, Berkelium, Beryllium, Bismuth, Boron, Bromine
- Cadmium, Calcium, Californium, Carbon, Cerium, Cesium, Chlorine, Chromium, Cobalt, Copper, Curium
- Dysprosium
- Einsteinium, Erbium, Europium
- Fermium, Fluorine, Francium
- Gadolinium, Gallium, Germanium, Gold
- Hafnium, Helium, Holmium, Hydrogen
- Indium, Iodine, Iridium, Iron
- Krypton
- Lanthanum, Lawrencium, Lead, Lithium, Lutetium
- Magnesium, Manganese, Mendelevium, Mercury, Molybdenum
- Neodymium, Neon, Neptunium, Nickel, Niobium, Nitrogen, Nobelium
- Osmium, Oxygen
- Palladium, Phosphorus, Platinum, Plutonium, Polonium, Potassium, Praseodymium, Promethium, Protactinium
- Radium, Radon, Rhenium, Rhodium, Rubidium, Ruthenium
- Samarium, Scandium, Selenium, Silicon, Silver, Sodium, Strontium, Sulfur
- Tantalum, Technetium, Tellurium, Terbium, Thallium, Thorium, Thulium, Tin, Titanium, Tungsten
- Uranium
- Vanadium
- Xenon
- Ytterbium, Yttrium
- Zinc, Zirconium
Frequency by First Letter of Name
AAAAA AA
BBBBB B
CCCCC CCCCC C
D
EEE
FFF
GGGG
HHHH
I
K
LLLLL
MMMMM
NNNNN NN
OO
PPPPP PPPP
RRRRR R
SSSSS SSS
TTTTT TTTTT
U
V
X
YY
ZZThe Element Symbols Alphabetically
Ac Ag Al Am Ar As At Au
B Ba Be Bi Bk Br
C Ca Cd Ce Cf Cl Cm Co Cr Cs Cu
Dy
Er Es Eu
F Fe Fm Fr
Ga Gd Ge
H He Hf Hg Ho
I In Ir
K Kr
La Li Lr Lu
Md Mg Mn Mo
N Na Nb Nd Ne Ni No Np
O Os
P Pa Pb Pd Pm Po Pr Pt Pu
Ra Rb Re Rh Rn Ru
S Sb Sc Se Si Sm Sn Sr
Ta Tb Tc Te Th Ti Tl Tm
U
V
W
Xe
Y Yb
Zn ZrThe Elements by Year Discovered or Isolated
- Gold, Copper, Silver, Lead, Iron, Tin, Carbon, Sulfur, Mercury, Arsenic, Antimony, Bismuth, Zinc (all ancient)
- Phosphorus (1669)
- Cobalt (1735), Platinum (1735)
- Nickel (1751), Magnesium (1755), Hydrogen (1766), Nitrogen (1772), Chlorine (1774), Manganese (1774), Oxygen (1774)
- Molybdenum (1778), Tellurium (1782), Tungsten (1783), Uranium (1789), Yttrium (1789), Zirconium (1789), Strontium (1790), Titanium (1791), Beryllium (1797), Chromium (1797)
- Niobium (1801), Vanadium (1801), Tantalum (1802), Cerium (1803), Iridium (1803), Osmium (1803), Palladium (1803), Rhodium (1803), Potassium (1807), Sodium (1807), Barium (1808), Boron (1808), Calcium (1808), Iodine (1811), Cadmium (1817), Lithium (1817), Selenium (1817), Silicon (1824)
- Aluminum (1825), Bromine (1826), Thorium (1829), Lanthanum (1839), Erbium (1842), Terbium (1843), Ruthenium (1844)
- Cesium (1860), Rubidium (1861), Thallium (1861), Indium (1863)
- Gallium (1875), Ytterbium (1878), Holmium (1879), Samarium (1879), Scandium (1879), Thulium (1879), Gadolinium (1880), Neodymium (1885), Praseodymium (1885), Dysprosium (1886), Fluorine (1886), Germanium (1886), Argon (1894), Helium (1895), Krypton (1898), Neon (1898), Polonium (1898), Radium (1898), Xenon (1898), Actinium (1899)
- Radon (1900), Europium (1901), Lutetium (1907), Protactinium (1913), Hafnium (1923)
- Rhenium (1925), Technetium (1937), Francium (1939), Astatine (1940), Neptunium (1940), Plutonium (1940), Americium (1944), Curium (1944), Promethium (1944), Berkelium (1949)
- Californium (1950), Einsteinium (1952), Fermium (1952), Mendelevium (1955), Nobelium (1958), Lawrencium (1961), Rutherfordium (1964), Dubnium (1967), Seaborgium (1974)
- Bohrium (1976), Meitnerium (1982), Hassium (1984), Darmstadtium (1994), Roentgenium (1994), Copernicium (1996), Flerovium (1998)
- Livermorium (2001), Moscovium (2004), Nihonium (2004), Oganesson (2006)
In symbols:
- Au, Cu, Ag, Pb, Fe, Sn, C, S, Hg, As, Sb, Bi, Zn (ancient or pre-1600)
- P (1669)
- Co (1735), Pt (1735)
- Ni (1751), Mg (1755), H (1766), N (1772), Cl (1774), Mn (1774), O (1774)
- Mo (1778), Te (1782), W (1783), U (1789), Y (1789), Zr (1789), Sr (1790), Ti (1791), Be (1797), Cr (1797)
- Nb (1801), V (1801), Ta (1802), Ce (1803), Ir (1803), Os (1803), Pd (1803), Rh (1803), K (1807), Na (1807), Ba (1808), B (1808), Ca (1808), I (1811), Cd (1817), Li (1817), Se (1817), Si (1824)
- Al (1825), Br (1826), Th (1829), La (1839), Er (1842), Tb (1843), Ru (1844)
- Cs (1860), Rb (1861), Tl (1861), In (1863)
- Ga (1875), Yb (1878), Ho (1879), Sm (1879), Sc (1879), Tm (1879), Gd (1880), Nd (1885), Pr (1885), Dy (1886), F (1886), Ge (1886), Ar (1894), He (1895), Kr (1898), Ne (1898), Po (1898), Ra (1898), Xe (1898), Ac (1899)
- Rn (1900), Eu (1901), Lu (1907), Pa (1913), Hf (1923), Re (1925)
- Tc (1937), Fr (1939), At (1940), Np (1940), Pu (1940), Am (1944), Cm (1944), Pm (1944), Bk (1949)
- Cf (1950), Es (1952), Fm (1952), Md (1955), No (1958), Lr (1961), Rf (1964), Db (1967), Sg (1974)
- Bh (1976), Mt (1982), Hs (1984), Ds (1994), Rg (1994), Cn (1996), Fl (1998)
- Lv (2001), Mc (2004), Nh (2004), Og (2006)
Frequency by Discovery Year
Count of 117 elements grouped by discovery year in 25-year periods.
Period | Count histogram
ancient | xxxxx xxxxx xxx
1650 | x
1725 | xx
1750 | xxxxx xx
1775 | xxxxx xxxxx
1800 | xxxxx xxxxx xxxxx xxx
1825 | xxxxx xx
1850 | xxxx
1875 | xxxxx xxxxx xxxxx xxxxx
1900 | xxxxx
1925 | xxxxx xxxxx
1950 | xxxxx xxxx
1975 | xxxxx xx
2000 | xxxxThallium and Other Poisons
Thallium (Tl) usually loses electrons to form cations. The two common oxidation (valence) states are:
- +1 (Tl⁺) → the thallous state
- +3 (Tl³⁺) → the thallic state
Now here’s the biological twist:
- Tl⁺ is very similar in size and charge to K⁺ (potassium), which is essential in nerve and muscle function. Cells can mistakenly take in Tl⁺ through potassium transport channels. Once inside, it disrupts critical potassium-dependent processes.
- Tl³⁺ is more reactive and less stable, but it can also form in compounds. The switching between +1 and +3 complicates how thallium behaves in the body.
So, “two valence states” means: thallium doesn’t behave consistently. In the +1 form it masquerades as potassium, sneaking into biology, and in the +3 form it can do additional damage. That dual chemistry is part of why it became notorious as a poison.
Here are other elements with multiple biologically confusing oxidation states.
- Cr = Chromium
- +3 (essential nutrient, part of glucose metabolism)
- +6 (toxic, carcinogenic, “Erin Brockovich” chromium)
- Mn = Manganese
- +2 (enzyme cofactor, essential)
- +3/+4/+7 (toxic at high levels; permanganate = strong oxidizer)
- Fe = Iron
- +2 (ferrous) and +3 (ferric) both common in biology.
- Iron constantly flips between them in hemoglobin, electron transport, etc.
- Cu = Copper
- +1 (cuprous) and +2 (cupric) both occur in enzymes.
- Misregulation is toxic (e.g. Wilson’s disease).
- Tl = Thallium
- +1 (mimics K⁺, sneaks into cells)
- +3 (more reactive, adds to toxicity)
- V = Vanadium
- +4 (vanadyl, VO²⁺) and +5 (vanadate, VO₃⁻) interconvert.
- Vanadate mimics phosphate in biology, confusing enzymes.
- As = Arsenic
- +3 (arsenite) and +5 (arsenate).
- Arsenate substitutes for phosphate in ATP reactions but fails, poisoning cells.
- Se = Selenium
- −2, +4, +6 states.
- Trace essential, but toxic at higher oxidation states.
- Mo = Molybdenum
- +4, +5, +6 states in enzymes (molybdenum cofactor).
- Needed for nitrogen metabolism.
Patterns
- Transition metals (Cr, Mn, Fe, Cu, Mo, V): multiple oxidation states are part of their usefulness in enzymes, but also a toxicity risk.
- P-block elements (As, Se, Tl): “mimicry poisons” — they resemble essential ions (P, S, K) and sneak into biochemistry.
Would you like me to present this as a table (element | states | biological role | toxic twist) so it slots straight into your blog post alongside the other lists?
Lavoisier’s Thirty Seven
In 1789, Antoine Lavoisier published his Traité Élémentaire de Chimie (Elementary Treatise of Chemistry), often considered the first modern chemistry textbook. In it, he presented a list of what he called éléments simples—fundamental substances that could not be decomposed by then-known chemical methods.
His list contained 33 substances, though in some later editions and translations it is sometimes described as 37 (depending on how one counts light, caloric, and variants). Here is the 1789 canonical list, grouped as he gave them:
Simple substances (imponderable fluids)
- Light
- Caloric (heat)
Simple substances (gases)
- Oxygen
- Azote (nitrogen)
- Hydrogen
Nonmetals
- Sulphur
- Phosphorus
- Charcoal (carbon)
- Diamond (carbon, again—he listed both separately)
Metals
- Antimony
- Silver
- Arsenic
- Bismuth
- Cobalt
- Copper
- Tin
- Iron
- Manganese
- Mercury
- Molybdenum
- Nickel
- Gold
- Platinum
- Lead
- Tungsten (scheelite)
- Zinc
Earths
- Lime (CaO)
- Magnesia (MgO)
- Barytes (BaO)
- Alumina (Al₂O₃)
- Silica (SiO₂)
His great achievement was not getting the list exactly right but systematizing chemical elements as basic building blocks, replacing alchemical notions.
His list contains twenty two elements:
O H S P C Sb Ag As Bi Co Cu Sn Fe Mn Hg Mo Ni Au Pt Pb W Zn
Multiple Elements from a Single Locality
Though nothing matches Ytterby’s seven, there are a few other spots where multiple elements trace back to a single locality. Geologically, the closest analogues to Ytterby are other rare-element pegmatites and alkaline intrusions, since both concentrate “incompatible” elements (those that don’t fit easily into common rock-forming minerals).
- Bastnäs, Sweden (Västmanland)
- Another pegmatite, discovered early 1800s.
- Source of bastnäsite, the mineral that gave name to the rare-earth family.
- Cerium (Ce) discovered here (1803 by Berzelius & Hisinger).
- Later yielded Lanthanum (La) and Didymium (later split into Neodymium (Nd) and Praseodymium (Pr)).
- Geological twin to Ytterby: coarse granite pegmatite enriched in rare earth oxides.
- Ilímaussaq complex, Greenland
- A rare large alkaline intrusion with exotic minerals like arfvedsonite, eudialyte.
- Source of sodium-rich minerals studied by Johan August Arfvedson (discovered Lithium, 1817).
- The mineral arfvedsonite (basis for Arfvedson’s discovery of Lithium) comes from related alkaline rocks.
- Also produced many type-locality minerals but fewer first-element discoveries.
- Famous for concentrating REEs, lithium, zirconium and still one of the world’s largest REE deposits.
- Minas Gerais, Brazil
- Classic granitic pegmatites, similar to Ytterby.
- Pegmatites rich in lithium, tantalum, niobium minerals.
- Early sources for Tantalum and Niobium (originally “columbium”).
- Also produced lithium minerals like spodumene and lepidolite.
- Kola Peninsula, Russia
- Complex of alkaline intrusions and pegmatites.
- Hosts exotic REE minerals (loparite, eudialyte) and was central to Soviet-era REE supply.
- Ontario & Quebec, Canada (e.g. Bancroft, Mont Saint-Hilaire)
- Pegmatites and syenite intrusions, yielding REE-bearing minerals (allanite, zircon, rare phosphates).
- Mont Saint-Hilaire is especially mineralogically rich, though less important historically for naming new elements.
- Joachimsthal (now Jáchymov, Czech Republic)
- A silver mining town in the Ore Mountains.
- Ores yielded Bismuth (studied here 16th c.), Cobalt, Nickel, and later Uranium (from pitchblende).
- Pitchblende from Jáchymov was what Becquerel and the Curies used to isolate Polonium and Radium.
- Cornwall, England
- Rich in tin and copper minerals, but also unusual species.
- William Gregor discovered Titanium (1791) from ilmenite in Cornwall.
- Humphry Davy worked there on alkali earths, isolating Magnesium, Calcium, Strontium, Barium (from Cornish minerals, though not all named for Cornwall).
- Ural Mountains, Russia
- Source of platinum-group elements: Platinum, Palladium, Rhodium, Osmium, Iridium, Ruthenium all traced back to alluvial deposits and ores studied in the 18th–19th centuries, see Section 19.
Geologically, Ytterby is not unique—though it is a “textbook” granitic pegmatite. What was unique is that it was quarried (for feldspar used in porcelain), close to universities, and mined at the exact historical moment when chemists were able to separate and recognize new elements.
Platinum Group Metals
The Ural Mountains (Russia) were the world’s main source of platinum and its relatives in the 19th century, and they played a huge role in discovering the platinum group elements (PGEs).
Geologically, the Urals host ultramafic rocks (peridotite, dunite, chromitite) emplaced during ancient tectonic collisions. PGEs are strongly compatible with sulfide and chromite, so they get concentrated in these rocks. Weathering then released platinum-rich grains into rivers → placer deposits (like alluvial gold).
Platinum (Pt) was known from South America earlier, but the Urals provided the first major commercial source in the early 1800s. From these ores, chemists in Europe isolated several “new metals” in quick succession:
- Palladium (Pd) – William Hyde Wollaston, 1803 (London; ore from South America originally, but Ural supply became important).
- Rhodium (Rh) – Wollaston, 1804.
- Iridium (Ir) & Osmium (Os) – Smithson Tennant, 1804 (from insoluble residues of platinum ores).
- Ruthenium (Ru) – Karl Ernst Claus, 1844 (Kazan University, Russia), specifically from Ural platinum concentrates.
By the 1820s, the Nizhny Tagil and Yekaterinburg districts were supplying virtually the world’s entire industrial platinum demand. Russia even issued platinum coins (1828–1845), minted from Ural platinum.The ores were “naturally alloyed” — mixtures of Pt with Pd, Ir, Os, and Ru — which is why they were so chemically puzzling.
References
- Greenwood & Earnshaw, Chemistry of the Elements (2nd ed., 1997)