ATP — Life’s Universal Energy Currency
Life is powered by tiny packets of energy that every living cell makes, uses, and remakes in an endless cycle. The most important of these packets is a molecule called ATP. Whether it is a bacterium deep in the ocean, a sunflower in a field, or a person running for a bus, ATP is the direct fuel that makes movement, growth, and repair possible. This post describes ATP and its role in life.
Notes created in conversation with GPT5.
What is ATP?
Adenosine triphosphate (ATP) is the molecule that powers life’s work. It is the primary chemical form of usable energy in all known organisms — from the simplest bacteria deep in the ocean to redwood trees and humans. After nucleic acids (DNA and RNA), ATP is arguably the single most important small molecule in biology.
The name is a clue to its structure: adenosine is made of adenine, a nitrogen-rich base, and ribose, a five-carbon sugar. Adenine comes from the Greek aden meaning “gland,” because it was first isolated from the pancreas. Ribose derives from “ribonic acid,” a sugar discovered in yeast; the “-ose” ending denotes sugars. “Triphosphate” means three phosphate groups attached — phosphate being a phosphorus atom bound to four oxygen atoms.
ATP is a small molecule; it’s tiny compared to the big biological macromolecules. In cell biology, molecules are often grouped broadly as:
Macromolecules — very large polymers made from repeating subunits, e.g.:
- Proteins (thousands of amino acids, ~10–100 kDa or more)
- Nucleic acids like DNA and RNA (millions of nucleotides)
- Polysaccharides (hundreds to thousands of sugar units)
Small molecules — typically <1–2 kDa, often just one or a few covalent “units” in size. They include:
- Metabolites (ATP, glucose, NADH)
- Signalling molecules (hormones, neurotransmitters)
- Cofactors (vitamins, coenzymes)
ATP is ~507 Da (about 500 g·mol\(^{-1}\)), which makes it a fraction of the mass of even a single small protein and roughly the size of a single amino acid tripeptide.
Calling it a “small molecule” sets it apart from the giants like DNA, but still acknowledges it has a complex, multi-part structure.
Discovery and Nobel Prizes
Two research groups reported the discovery of ATP in 1929. Cyrus H. Fiske and Yellapragada Subba Row at Harvard Medical School (Boston) isolated it from mammalian muscle and liver. Likewise, Karl Lohmann at the Kaiser Wilhelm Institutes (Berlin and Heidelberg) identified it in muscle tissues.
It was first synthesized in the laboratory by Alexander Todd in 1948, and he was awarded the Nobel Prize in Chemistry in 1957 partly for this work.
The 1978 Nobel Prize in Chemistry was awarded to Peter Dennis Mitchell for the discovery of the chemiosmotic mechanism of ATP synthesis.
The Nobel prize for Chemistry in 1997 was been shared by
- Dr John Walker of the Medical Research Council’s Laboratory of Molecular Biology (LMB) at Cambridge,
- Dr Paul Boyer of the University of California at Los Angeles, and
- Dr Jens Skou of Aarhus University in Denmark.
The prize was for the determination of the detailed mechanism by which ATP shuttles energy. The enzyme which makes ATP is called ATP synthase, or ATPase, and sits on the mitochondria in animal cells or chloroplasts in plant cells. Walker first determined the amino acid sequence of this enzyme, and then elaborated its 3-dimensional structure. Boyer showed that contrary to the previously accepted belief, the energy requiring step in making ATP is not the synthesis from ADP and phosphate, but the initial binding of the ADP and the phosphate to the enzyme. Skou was the first to show that this enzyme promoted ion transport through membranes, giving an explanation for nerve-cell ion transport as well as fundamental properties of all living cells. He later showed that the phosphate group that is ripped from ATP binds to the enzyme directly. This enzyme is capable of transporting sodium ions when phosphorylated like this, but potassium ions when it is not.
Nucleotides
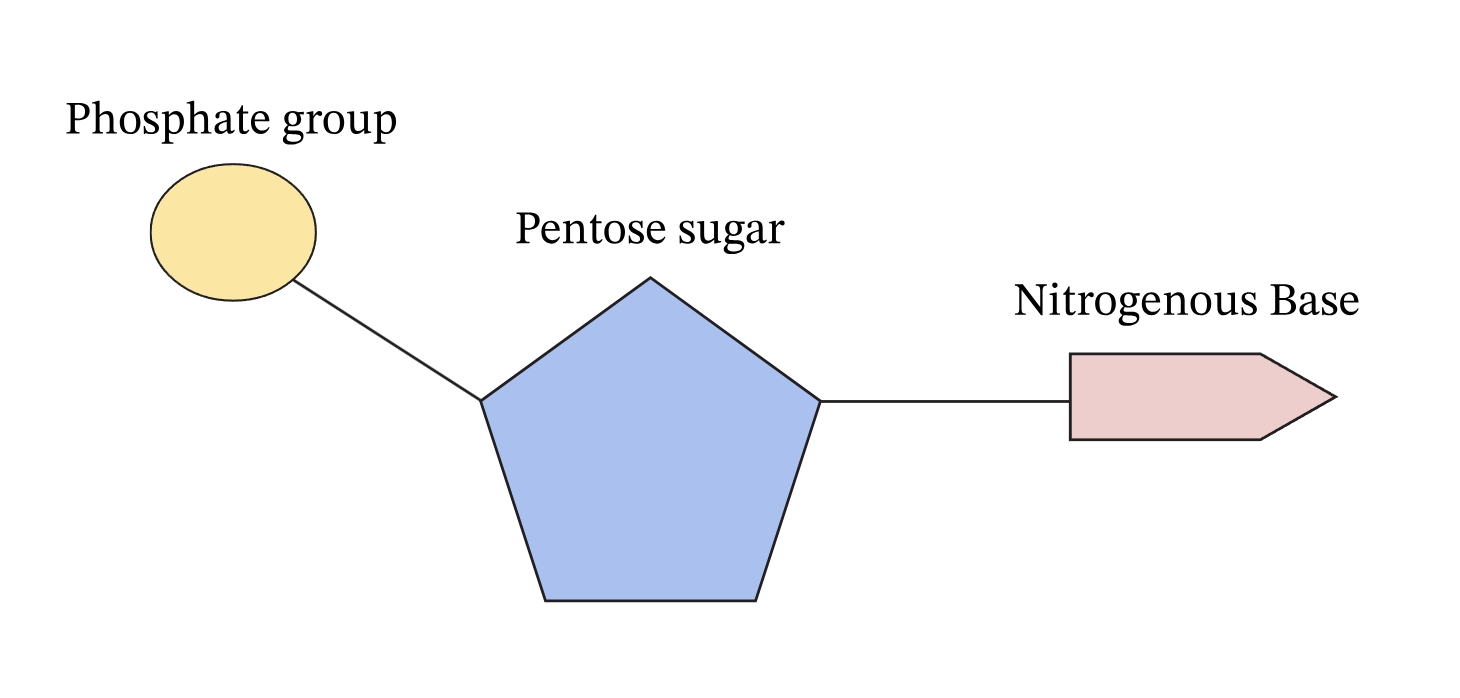
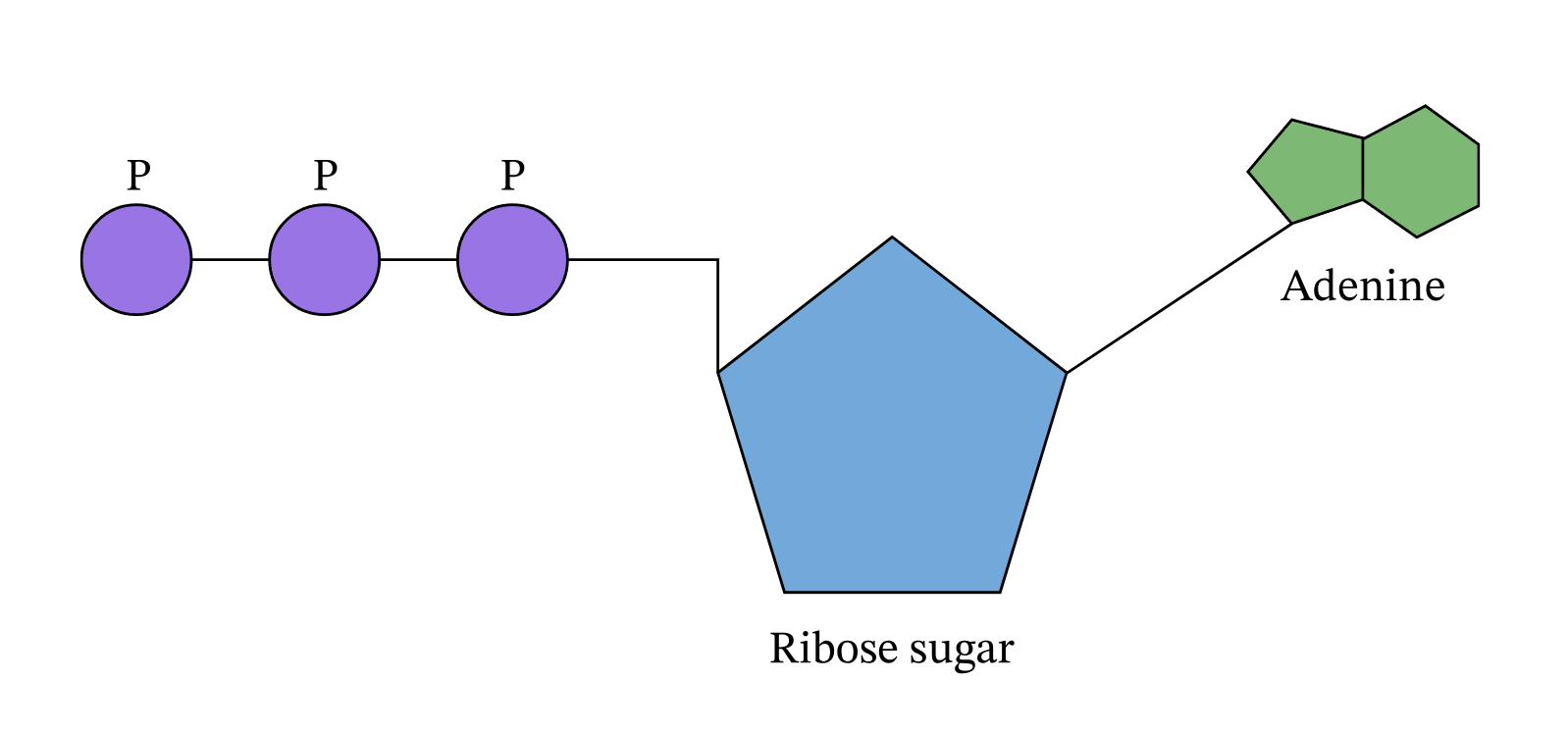
ATP is, in fact, a nucleotide — the same type of basic molecular unit that makes up DNA and RNA. We often meet the term “nucleotide” in a genetic context, but it is broader than that: nucleotides are small, versatile molecules built from three parts — a nitrogen-containing base, a five-carbon sugar, and one or more phosphate groups. In ATP’s case, the base is adenine, the sugar is ribose, and there are three phosphate groups — hence adenosine triphosphate. This simple arrangement is what gives ATP its remarkable role as life’s universal energy currency.
Chemical Structure
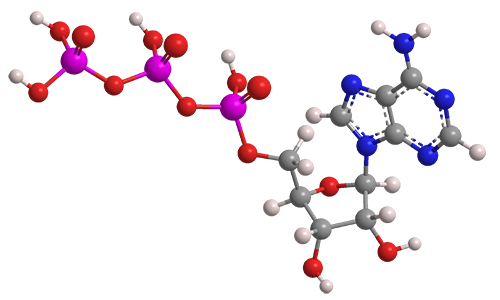
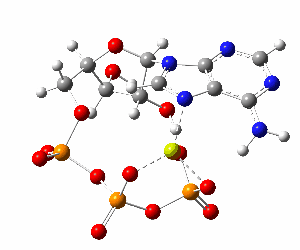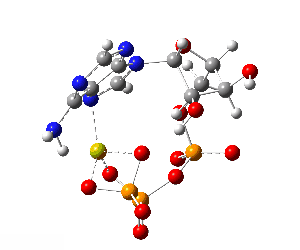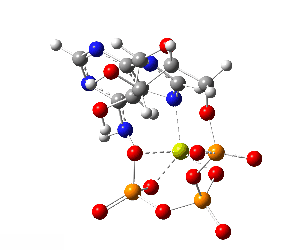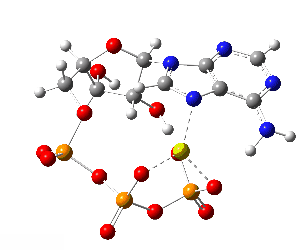
ATP’s chemical formula is \(\mathrm{C_{10}H_{16}N_{5}O_{13}P_{3}}\), molar mass about 507.18 g·mol\(^{-1}\). It has three parts:
- Adenine – a nitrogenous base (a flat, aromatic ring structure containing nitrogen atoms that can accept protons and donate electron pairs).
- Ribose – a five-carbon sugar that links the adenine to the phosphate tail.
- Three phosphate groups – linked by phosphoanhydride bonds.
These bonds are “high-energy” not because they are unusually strong, but because breaking them via hydrolysis yields products — ADP and inorganic phosphate — that are more stable and strongly hydrated.
Inorganic phosphate is denoted \(P_i\). The “inorganic” means it is not bound into a larger organic molecule; in cells it is free-floating in solution as \(\mathrm{HPO_4^{2-}}\) or \(\mathrm{H_2PO_4^-}\).
Although the phosphate tail is the part of ATP that releases usable energy, the rest of the molecule — the ribose sugar and adenine base — is far from decorative. Linking the phosphates to ribose–adenine makes the molecule more stable in water than a bare chain of phosphates, which would otherwise be prone to uncontrolled breakdown. The base–sugar portion also acts as a universal molecular “handle” that enzymes can recognize and bind. Because countless enzymes have evolved to work with nucleotides, this shared structure allows ATP to fit seamlessly into a vast range of biochemical processes without each one needing its own binding system. The nucleotide form also integrates ATP into other parts of metabolism: it can serve as a building block for RNA and, in modified forms, for DNA, linking energy metabolism to genetic chemistry. In addition, the adenine–ribose segment keeps ATP soluble, makes it easier to regulate, and enables specific interactions with proteins. Longer phosphate chains — adenosine tetraphosphate, pentaphosphate, and beyond — do exist in nature, but they are rare, unstable, and serve only niche functions. Their high negative charge makes them harder to handle, and the energy yield per phosphate does not justify the extra chemical difficulty. For most of life’s needs, the triphosphate form is the optimal balance of energy content, stability, and compatibility with the cell’s enzyme systems.
In the Brønsted–Lowry sense:
- Acid → donates a proton (H⁺)
- Base → accepts a proton (H⁺)
In the Lewis sense:
- Acid → accepts an electron pair
- Base → donates an electron pair
Adenine is called a nitrogenous base because its nitrogen atoms can accept protons and donate electron pairs, which also lets it form hydrogen bonds in DNA and RNA pairing.
Why ATP Has the Shape It Does
Although the phosphate tail is the part of ATP that releases usable energy, the rest of the molecule — the ribose sugar and adenine base — is far from decorative. Linking the phosphates to ribose–adenine makes the molecule more stable in water than a bare chain of phosphates, which would otherwise be prone to uncontrolled breakdown. The base–sugar portion also acts as a universal molecular “handle” that enzymes can recognize and bind. Because countless enzymes have evolved to work with nucleotides, this shared structure allows ATP to fit seamlessly into a vast range of biochemical processes without each one needing its own binding system. The nucleotide form also integrates ATP into other parts of metabolism: it can serve as a building block for RNA and, in modified forms, for DNA, linking energy metabolism to genetic chemistry. In addition, the adenine–ribose segment keeps ATP soluble, makes it easier to regulate, and enables specific interactions with proteins.
Longer phosphate chains — adenosine tetraphosphate, pentaphosphate, and beyond — do exist in nature, but they are rare, unstable, and serve only niche functions. Their high negative charge makes them harder to handle, and the energy yield per phosphate does not justify the extra chemical difficulty. For most of life’s needs, the triphosphate form is the optimal balance of energy content, stability, and compatibility with the cell’s enzyme systems.
Building ATP from Scratch
While most ATP in cells comes from recycling ADP, organisms can make it from simpler building blocks. Adenine is assembled by the purine synthesis pathway from amino acids like glycine, aspartate, and glutamine plus formate and CO₂. Ribose comes from glucose via the pentose phosphate pathway. Phosphate groups come from dietary phosphate or from breaking down other phosphate-containing molecules. These components are joined to form adenosine monophosphate (AMP), then phosphorylated to ADP and finally ATP.
Systems for Producing ATP
Once the basic components exist, cells generate ATP continuously by adding phosphate to ADP using energy from an external source. The main routes are:
- Oxidative phosphorylation — in mitochondria and many bacteria, energy from oxidizing glucose, fatty acids, or amino acids is used to pump protons across a membrane.
- Photophosphorylation — in chloroplasts and cyanobacteria, light excites electrons in pigments, starting the proton-pumping chain.
- Substrate-level phosphorylation — a phosphate is transferred directly from a high-energy intermediate to ADP (as in glycolysis).
- Ion-driven phosphorylation — in some archaea, ATP synthase runs on sodium ion gradients instead of protons.
Role in Respiration
Respiration is the set of processes that harvest energy from electron flow to generate a proton (or sodium) gradient, which drives ATP formation.
- Aerobic respiration (aerobic from Greek aēr, “air,” and bios, “life”) uses oxygen (\(\mathrm{O_2}\)) as the final electron acceptor.
- Anaerobic respiration (an- = “without”) uses alternatives like nitrate (\(\mathrm{NO_3^-}\)), sulfate (\(\mathrm{SO_4^{2-}}\)), or ferric iron (\(\mathrm{Fe^{3+}}\)).
- Fermentation produces ATP without an electron transport chain, directly transferring phosphate from a substrate to ADP.
- Chemolithotrophy (“rock eating”) oxidizes inorganic compounds such as hydrogen gas (\(\mathrm{H_2}\)), ammonia (\(\mathrm{NH_3}\)), or ferrous iron (\(\mathrm{Fe^{2+}}\)) to drive the gradient-forming machinery.
In every case, the endpoint is the same: ADP + \(P_i\) + energy → ATP.
ATP synthase is a rotary enzyme embedded in membranes: the inner mitochondrial membrane in animals, thylakoid membranes in plants, and plasma membranes in many microbes.
Respiration or photosynthesis pumps protons to one side of the membrane, creating a gradient. As protons flow back through ATP synthase, they turn a rotor in the membrane sector (F₀). This mechanical rotation is transmitted to the catalytic sector (F₁) inside the cell, where it changes shape in three repeating sites: one binds ADP and \(P_i\), one squeezes them together into ATP, and one releases ATP. The protons are not part of the ATP — they supply the torque that drives the cycle.
Role as an Energy Provider
When ATP is hydrolyzed (hydro = water, lysis = breaking), the terminal phosphate is removed: \[ \mathrm{ATP + H_2O \longrightarrow ADP + P_i + energy}. \] The energy release — about 50 kJ·mol\(^{-1}\) under cellular conditions — comes from relief of electrostatic repulsion, more stable products, and strong hydration of the products.
One molecule releases about \(8.3 \times 10^{-20}\) J, but a mole (6.022 × 10²³ molecules) releases ~50 kJ — roughly the energy to lift a 100 g apple 5 m. A mole of ATP is about 507 g; the body only has ~0.2 mol (100 g) at any moment, so the real power comes from turnover speed, not stored mass.
An aromatic ring is a flat loop of atoms with a special electron arrangement: π-electrons (electrons in p-orbitals above and below the ring) are delocalized over the whole structure. This delocalization makes the ring unusually stable. In adenine, the aromatic rings contain carbon and nitrogen atoms, giving rigidity and helping it stack with other bases in DNA and RNA. The π-electrons also influence light absorption and molecular recognition.
Energy from a Single ATP Breakdown
Breaking one molecule of ATP to ADP + \(P_i\) releases roughly \(8.3 \times 10^{-20}\) joules under typical cellular conditions.
That is unimaginably small on its own — but multiply by a mole (6.022 × 10²³ molecules) and you get about 50 kJ. That’s roughly:
- The energy needed to lift a small apple (100 g) about 5 m straight up.
- The same as the energy in about 1.2 nutritional kilocalories (food calories).
Even though the body only contains ~100 g of ATP at a given moment, it is recycled extremely fast — each molecule is reused hundreds to thousands of times per day. The total daily ATP turnover for a resting adult is in the range of 40–70 kg, and can be over 100 kg with vigorous activity.
It is not “energy dense” in the same way as petrol or fat — it’s not a long-term store — but its instant availability and rapid regeneration make it the perfect short-term energy currency. The real “density” comes from the turnover rate: you keep spending and replenishing that 100 g every few minutes.
Scale of Turnover
A typical human cell cycles \(10^7\)–\(10^8\) ATP molecules every second. With about \(1.2 \times 10^{13}\) cells in the body, that’s on the order of \(6 \times 10^{20}\) molecules per second, or ~0.001 mol per second — about 50 watts of continuous power just from ATP turnover at rest. Total daily turnover is about 40–70 kg of ATP equivalents, rising over 100 kg in sustained exercise.
Plants vs. Humans
Plants make ATP in two places: chloroplasts (light-driven, for CO₂ fixation) and mitochondria (oxidative, for general metabolism). Humans make almost all ATP in mitochondria, except in cells without them (like red blood cells), which depend entirely on glycolysis. In both, ATP is the final, universal currency for energy transactions.
Here’s a Markdown table you can drop straight after the Systems for Producing ATP section. It summarizes the main ATP production routes across life, showing inputs, mechanism, location, and examples.
ATP Production Routes Across Life
| Route | Energy Source | Electron / Proton Gradient Driver | Final Electron Acceptor | Location (in cell) | Typical Organisms |
|---|---|---|---|---|---|
| Oxidative phosphorylation | Oxidation of organic molecules (glucose, fatty acids, amino acids) | Electron transport chain pumps protons (\(\mathrm{H^+}\)) | \(\mathrm{O_2}\) (aerobic) or alternative acceptors (anaerobic, e.g., \(\mathrm{NO_3^-}\), \(\mathrm{SO_4^{2-}}\), \(\mathrm{Fe^{3+}}\)) | Inner mitochondrial membrane (eukaryotes), plasma membrane (prokaryotes) | Animals, plants, fungi, many bacteria and archaea |
| Photophosphorylation | Sunlight | Light-excited electrons in pigments pump protons | \(\mathrm{NADP^+}\) (reduced to NADPH) or cyclic flow | Thylakoid membranes in chloroplasts (plants, algae), thylakoid-like membranes (cyanobacteria) | Plants, algae, cyanobacteria |
| Chemolithotrophy | Oxidation of inorganic compounds (e.g., \(\mathrm{H_2}\), \(\mathrm{NH_3}\), \(\mathrm{Fe^{2+}}\), \(\mathrm{H_2S}\)) | Specialized electron transport chain pumps protons | Often \(\mathrm{O_2}\), but can be nitrate, sulfate, ferric iron | Plasma membrane | Bacteria, archaea (deep-sea vent microbes, nitrifying bacteria) |
| Substrate-level phosphorylation | High-energy intermediate in metabolic pathway (e.g., 1,3-bisphosphoglycerate in glycolysis) | None — direct phosphate transfer to ADP | N/A (no electron transport) | Cytoplasm (glycolysis), mitochondrial matrix (citric acid cycle) | All organisms (especially important in anaerobes and in cells without mitochondria) |
| Sodium-driven phosphorylation | Ion gradient of \(\mathrm{Na^+}\) instead of \(\mathrm{H^+}\) | Sodium motive force across membrane | Variable (depends on upstream energy source) | Plasma membrane | Some archaea and bacteria |
Closing
ATP is not the only molecule that carries energy — others include GTP, NADH, and reduced ferredoxin — but none match its universality. Life’s deep chemistry is a flow of electrons and protons captured in gradients, stored briefly in ATP, and spent in countless cellular processes. It is the cash of life’s economy: small, versatile, spent quickly, and earned again just as fast.